Bismuth (Bi), element number 82 on the periodic table, does not play by the rules. This metal is dense when the laws of nature suggest it should be loose, contracts when it should expand and repels magnets like no other metal does. Only an element so contradictory could be found in both fireworks and a soothing medical treatment.
There is almost twice as much bismuth as gold in the Earth’s crust.
We often think of metals as being solid, heavy, tough materials that are very hard to bend or break. Bismuth, on the other hand, is soft, brittle and easily altered.
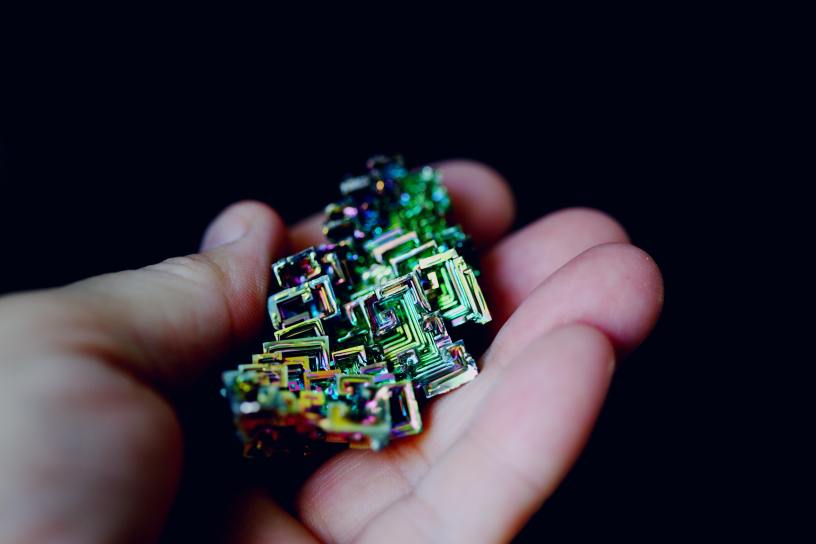
A crystalline, white metal with a slight pink tinge, bismuth is a solid at room temperature. It doesn’t take much heat to melt it, however, and that is when bismuth truly shines. As it cools down from its melted state, it reorganizes its molecules into remarkable shapes, creating crystals much like water does when it creates a snowflake. Bismuth crystals climb and twist like tiny spiral staircases, their outer edges growing at a faster rate than their inner ones.
Further enhancing the beauty of the bismuth crystal is when it oxidizes (when metal is exposed to oxygen). You may be familiar with the red-orange rust that forms when iron oxidizes. When bismuth oxidizes all colours of the rainbow spring forth in a dazzling display.
Bismuth resists being magnetized and will create its own magnetic field by altering the orbit of its electrons to repel the magnetic field of another object. This characteristic is called diamagnetism and bismuth is the single most naturally diamagnetic metal or element known.
The word “bismuth” comes from an Old German word, “weissmuth” or “white substance.”

As far back as ancient Egypt, bismuth has been an additive in many cosmetics including lipsticks, eyeshadow and nail polish, its pearly powder making the products shine. It is also found in popular treatments for stomach aches and diarrhea, such as Pepto-Bismol.
Bismuth gives popular fireworks displays their sizzle. It can be found in the firework effect known as the “Dragon’s Egg” and in sparklers, lending a nice crackling sound to the spectacle.
Bismuth’s low melting point is ideal for use in sprinkler systems. The heat rising to the ceiling in a burning building will quickly warm and melt a bismuth plug which releases to dowse the flames with water.
Unlike most metals when bismuth freezes it expands rather than contracts. It also denser as a liquid than a solid.
Because of its low toxicity bismuth has been found to be an environmentally friendly substitute for lead, an element it was originally mistaken for. You can find it in pencils, plumbing, fishing weights, ammunition, and other products where lead was originally used.
Article originally published in Brainspace Magazine in Summer 2023.